Residual Chlorine Monitoring
Pi has three residual chlorine analysers for free and total chlorine. These are the HaloSense based on the electrochemical technology, the DPDSense (an online DPD analyser) and the ChloriBrid (hybrid, innovative and patented XXXXX. Both technologies that bring the benefits of both to a single Chlorine analyser.
Residual chlorine analysers from Pi are used in many applications requiring the measurement and control of online residual chlorine levels in water. The range is suitable for total or free residual chlorine monitoring or control applications in potable water, seawater, process water, swimming pool water, wastewater, food washing, paper and pulp, etc.
The Pi range of controllers/transmitters means that you get exactly what you need and nothing that you don’t. From a low cost no-frills chlorine dosing controller (CRONOS®) to a highly sophisticated colour display, remote access controller (CRIUS®4.0) – and all with the same great sensors! Chlorine dosing control is now simpler and cheaper than ever! Both controllers can have multiple sensors and multiple sensor types, saving money on the requirement for one sensor and one transmitter per measurement.
Online electrochemical is an online DPD method of measuring residual choline has different strengths and weaknesses, so depending on your application you maybe better off with an electrochemical solution (HaloSense) or online DPD solution (DPDSense) or if you need the benefits of both the hybrid ChloriBrid will be the instrument of choice for you.
Benefits | HaloSense | DPDSense | ChloriBrid |
|---|---|---|---|
Instant start up | X | ✓ | ✓ |
Continuous measurements | ✓ | X | ✓ |
No chemical reagent | ✓ | X | X |
Instant recovery from power outage | X | ✓ | ✓ |
When chlorine is added as a disinfectant to water it oxidises material in the water thereby killing any organisms. The ‘Residual Chlorine’ is the chlorine left over at the end of the process and is usually what we measure.
Free chlorine is the chlorine in the water that exists as HOCl or OCl–. When chlorine is added to pure water between pH 4 and pH 11
Cl2 + OH– ↔ HOCl + Cl–
HOCl ↔ OCl– + H+
so if chlorine is added to water you get HOCl (Hypochlorous acid) and OCl– (Hypochlorite), which together make ‘free chlorine’.
If water contains both ammonia and hypochlorite it will react to form monochloramine.
NH3 + OCl– → NH2Cl + OH–
In an acidic solution Monochloramine disproportionates to form Nitrogen Trichloride.
2NH2Cl + H+ → NHCl2 + NH4+
3NHCl2 + H+ → 2NCl3 + NH4+
In solution where there are low concentrations of chlorine it is often Chloramines that can be smelled not ‘chlorine’. The three Chloramines above are collectively known as ‘Combined Chlorine’.
No. Total Chlorine is the sum of Free Chlorine and Combined Chlorine, therefore Total Chlorine is always equal to or greater than both Combined Chlorine or Free Chlorine.
Focus Ons are a series of short articles distributed by email providing technical information regarding instrumentation, process measurement in potable, waste, process and pool waters. If you would like to join the mailing list, please contact us.
For many years, online DPD measurement of free chlorine has been seen as the most reliable, and best way, to ensure an accurate measurement of free chlorine in water. However, over the past 20 years, huge advances have been made in amperometric measurements with little or no advancements made in DPD measurement technology, so is online DPD still the best way to measure chlorine in water?
Did you know that…
…Pi’s amperometric chlorine probes only require maintenance once a year?
…Pi’s amperometric range of probes do not use reagents and therefore have no ongoing running costs?
…a utility company saved £millions when they swapped from online DPD to Pi’s amperometric free chlorine probes?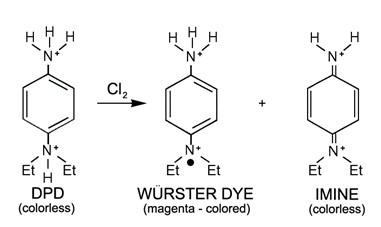
DPD based systems
This technology uses the chemical reaction between DPD (N.N-diethyl-p-phenylenediamine) and an oxidant, e.g. free chlorine, to measure the oxidant level. The chemical reaction causes a visible change from colourless to pink, which is proportional to the level of oxidant.
Free chlorine is measured by adding a precisely measured amount of DPD reagent to the sample, along with a pH stabilising buffer to maintain the pH between 6.3 and 6.6. Total chlorine can be measured by adding iodine and reducing the pH to 5.1.
The DPD reaction requires precise measurement of the DPD chemical, sample volume and buffer to ensure that the reaction is reproducible and accurate. To do this, DPD based online systems require very accurate dosing measurements and fine tubing to carry the precise volumes. To keep a DPD based online system working correctly, it is essential that the instrument is serviced annually with all tubing replaced. The reagents used in the DPD chemical reaction also require monthly replacement which, when combined with the cost of the service kit and time taken to complete the maintenance tasks, means that the ongoing maintenance costs are much more than the instrument purchase price. As many companies are taking more notice of total costs (Totex) rather than just the initial equipment costs (Capex), DPD based online systems are beginning to look less appealing.
Amperometric based systems
These use electrochemistry to measure the chlorine concentration. The current produced by the reduction of chlorine is measured and this current is proportional to the amount of chlorine present. Amperometric probes can measure free or total chlorine. Many other oxidants such as peracetic acid, chlorine dioxide and ozone can be measured with oxidant specific amperometric probes.
Traditionally, amperometric probes required 2-point calibrations, regular cleaning, pH compensation/pH buffering and very precise flow control. This meant that they required lots of maintenance or didn’t give accurate results. This is no longer the case.
Amperometric probes have gone through multiple technological changes over the past 20 years and the result of these means that modern day amperometric probes suffer from none of these issues. Amperometric probes that utilise all of the technological discoveries, like the ones supplied by Pi, only require single point calibration because zero drift has been completely eradicated.
In clean applications, they require no cleaning. In dirty applications, Pi can provide automatic flushing systems to keep the sensor clean and functioning. This is all down to new membrane materials. They do not require pH compensation or buffering except in the most extreme and variable pH applications, and flow variations have been minimised to a negligible level due to improved flow cell designs. All of these improvements mean that the advantages once enjoyed by DPD measurement technologies are now non-existent and amperometric technology is quite understandably becoming the standard in nearly all applications.
Case Study – Savings made when switching from DPD to amperometric measurement
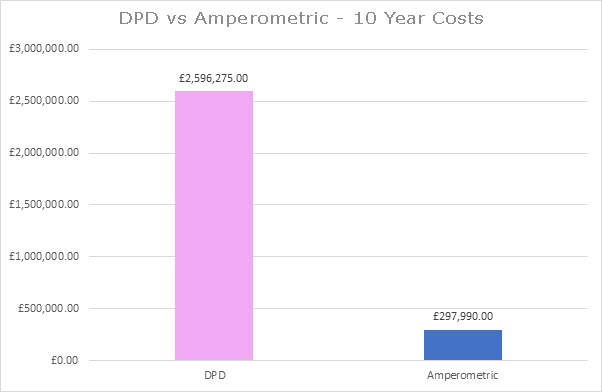 More than ten years ago, a large UK water utility company made the decision to remove all of their DPD based, chlorine measurement instruments and replace them with Pi’s HaloSense amperometric analysers. This puts Pi in a unique position to accurately calculate the savings made by this water company as a direct result of this change.
More than ten years ago, a large UK water utility company made the decision to remove all of their DPD based, chlorine measurement instruments and replace them with Pi’s HaloSense amperometric analysers. This puts Pi in a unique position to accurately calculate the savings made by this water company as a direct result of this change.
330 DPD based instruments were removed and Pi’s HaloSense chlorine systems were installed over a period of 6 months. The first graph shows the calculated savings made by this UK water company over ten years using the current list prices for maintenance parts and reagents. This shows that, at current prices, savings of £2.3million would have been made over this ten year period. The figures don’t include the savings made through the reduction of monthly DPD replacement visits.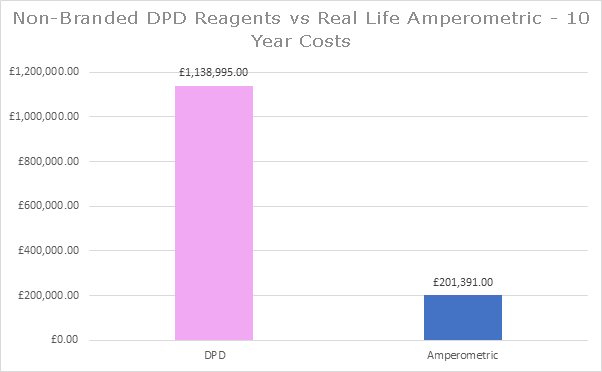 It is possible to purchase non-branded reagents for DPD based systems which does reduce the ongoing cost of these systems. However, real world data of the 330 amperometric systems installed also shows that maintenance times of the installed amperometric probes could be extended in most cases, well beyond the recommended frequency, which also reduces the cost of the amperometric systems. Taking both of these factors into account, the second graph shows the adjusted savings which, in this real world example, still adds up to £937,000.
It is possible to purchase non-branded reagents for DPD based systems which does reduce the ongoing cost of these systems. However, real world data of the 330 amperometric systems installed also shows that maintenance times of the installed amperometric probes could be extended in most cases, well beyond the recommended frequency, which also reduces the cost of the amperometric systems. Taking both of these factors into account, the second graph shows the adjusted savings which, in this real world example, still adds up to £937,000.
Coupling an amperometric probe with Pi’s CRIUS®4.0 controller makes it possible to not only reduce maintenance costs but to also reduce emergency callouts and unscheduled maintenance work by utilising the CRIUS®4.0 pro-active maintenance warnings and remote communications, whilst also improving the overall quality of measurement results.
Membraned sensors come with many advantages over non-membraned sensors such as higher resolutions, fewer interferents and a greatly reduced effect of flow rate changes. These advantages can make a huge difference to the bottom line, particularly if the cost of the chemical being dosed is quite high. For free chlorine sensors, using a membrane can make your measurement much less dependent on pH (if you are using sensors from Pi), meaning your measurement is a more accurate reflection of chlorine residual.
As such, membraned sensors are now largely the norm in residual chlorine measurement and are also prevalent for chlorine dioxide and ozone monitors, but did you know that…
…membraned sensors are sensitive to changes in pressure?
…flow cell outlets can airlock even when water is flowing through them?
…membraned sensors can still be used when the outlet does not go to drain?
…Pi has engineered and designed solutions to all of these potential issues?
Sensitivity to pressure
Membraned sensors do have one property that needs to be carefully managed; they are sensitive to pressure. Pi was an early adopter of membrane technology, so we know that the installation of these sensors is just as important as the sensor itself. In fact, the same sensors in different flow cells can give very different results.
In order to prevent pressure variations affecting the probe, Pi typically uses open flow cells which eliminate variability in pressure before it reaches the probe.
Flow
Whether the sample to the cell is pumped, gravity fed, or comes from a pressurised line, it is important that the flow is controlled to within a range of 350-1000ml per minute, to ensure that sufficient flow is reaching the sensor and to prevent the flow cell overflowing.
If the flow to the cell is variable, Pi can provide a dole valve which controls the flow to approximately 500ml per minute, which prevents the cell from overflowing when pressure variations mean more flow than the cell can handle, while also ensuring adequate flow when the sample line flow/pressure reduces.
Airlocks
The outlet of the flow cell needs to be open to atmosphere, and completely unobstructed. Any system with a long outlet line (particularly flexible pipe) is prone to get airlocks, which will cause the cell to overflow. Outlets which are visually clear and even have water flowing through them, can be partially airlocked which causes backpressure to overflow the cell. This is very easy to diagnose as if you see the cell overflowing and remove the outlet pipe, you will see the cell go back to normal operations within approximately 10 seconds. If this is a persistent problem, consider putting in an air break using a commercially available tundish.
Outlets that do not go to drain
The water from a membraned sensor doesn’t have to go to drain. For processes where saving water is a high priority, a simple tank and pump system that will pump sample water back into your main process line will allow water losses to be reduced to almost zero. Pi’s CRIUS®4.0 controller can be used to control this return process and ensure that this tank never overflows and can automatically drain itself periodically to avoid sediment build up.
What if things go wrong?
As any water engineer can tell you, no matter how well the system is designed, lines can clog, pumps can break and someone on site could fiddle with the settings. Pi recognises these challenges and has engineered solutions into our systems. All Pi membraned sensors have the option to be able to:
- Have a flow switch to detect sample flow loss.
- Use dosing overfeed protection to protect against clogged dosing lines or pump failure.
- Have remote access for SMS or email alarms.
- Use relays to trigger beacons or sirens for alarms or control valves and pumps.
- Customise user security levels to control who can change what settings.
- Use status logs that show what happened to the system and when.
Closed Flow Cells
For membraned sensors, the best way of housing a membraned sensor is with an open flow cell. There are some occasions where this solution just isn’t practicable and in those instances the closed flow cells from Pi, which can take an overpressure of up to 3 bar, are the best solution.
Did you know that…
…when you dose chlorine into seawater it is bromine that does the disinfection?
…DPD 1 measures free chlorine or total bromine and not free bromine?
The chemistry of the chlorination of seawater is more complex than many people realise and, although the measurement of chlorine residuals is possible in seawater (and therefore automatic control of chlorine dosing), better results will be obtained if this is fully understood.
Is it Chlorine or is it Bromine?
 Seawater contains about 65-80ppm dissolved bromides1 most of which are sodium bromide. When you put chlorine in water it displaces the bromine from the bromide (because it’s more reactive) and becomes a chloride. So for up to about 70ppm of total chlorine dosed, what you actually have in the water is free bromine and combined bromine (NOT free and combined chlorine), so it is the total bromine that actually does the disinfection2. So why does everyone call it chlorination when technically it is bromination? Mainly because most people don’t know this interesting bit of chemistry. So what? Normally it makes no difference as total bromine is an effective disinfectant, however there can be a lot of confusion when it comes to monitoring residuals and controlling dosing. Choosing the correct sensor to control the dosing is crucial as is choosing the correct DPD test.
Seawater contains about 65-80ppm dissolved bromides1 most of which are sodium bromide. When you put chlorine in water it displaces the bromine from the bromide (because it’s more reactive) and becomes a chloride. So for up to about 70ppm of total chlorine dosed, what you actually have in the water is free bromine and combined bromine (NOT free and combined chlorine), so it is the total bromine that actually does the disinfection2. So why does everyone call it chlorination when technically it is bromination? Mainly because most people don’t know this interesting bit of chemistry. So what? Normally it makes no difference as total bromine is an effective disinfectant, however there can be a lot of confusion when it comes to monitoring residuals and controlling dosing. Choosing the correct sensor to control the dosing is crucial as is choosing the correct DPD test.
Free Chlorine and Total Bromine

Due to the confusion on what is being measured, it is easy for an engineer to specify the wrong equipment and calibrate it incorrectly. For example, it is common for a free chlorine sensor to be specified for seawater chlorination control. Most electrochemical free chlorine sensors will react to free bromine (not all so be careful!) but this isn’t necessarily what you need for bromination control. Most authors agree that whilst the disinfection capability between free chlorine and combined chlorine differs, when it comes to free bromine and combined bromine, both forms of the chemical are equally good at disinfection so a better measurement would be total bromine, which requires a total bromine sensor.
DPD and Seawater Chlorination
To add to this already confusing environment we need to look at calibrating online sensors. DPD is used extensively to measure chlorine residuals and it also reacts to bromine so can be used for both, however, DPD 1 measures FREE chlorine or TOTAL bromine. The situation can therefore arise where you have an online instrument such as a CRONOS® or CRIUS® specified as a free chlorine, actually measuring free bromine but calibrated as a total bromine (against DPD 1)! Typically, the best results are obtained by specifying a total bromine (total chlorine) sensor and calibrating it using DPD 1.
Estuarine Waters
Many seawater chlorination applications are estuarine in nature (partly seawater and partly fresh water) and it is the degree of dilution which determines which sensor and which electrolyte you should use. Seawater has approximately 70ppm bromides and so up to 70ppm chlorine the replacement will be 100%. If the seawater is 50% fresh water then up to 35ppm chlorine will give 100% displacement. For example, if we looked at a 2ppm residual then the water could be only 3% seawater and 97% fresh water and you would still be measuring bromine, so a total bromine sensor calibrated with DPD 1 would be appropriate. Because bromine is heavier than chlorine, 1mg/l of chlorine is the same as approximately 1.6mg/l of bromine3 so it is important to understand what you are measuring. DPD kits can be used in three ways for the chlorination of seawater.
Because bromine is heavier than chlorine, 1mg/l of chlorine is the same as approximately 1.6mg/l of bromine3 so it is important to understand what you are measuring. DPD kits can be used in three ways for the chlorination of seawater.
- A chlorine DPD 1 kit will measure total bromine but will report the concentration as chlorine equivalent. If this is used to calibrate a Pi sensor, it should be a total chlorine probe that will also then report in mg/l of chlorine equivalents.
- A chlorine DPD 1 test kit can be used and the mg/l can be multiplied by 1.6 to give a total bromine reading, and if this is used to calibrate a Pi sensor it should be a total bromine sensor.
- A bromine DPD 1 test kit is the same as a chlorine test kit except that it does multiply internally and outputs as mg/l of total bromine. If the kit is used to calibrate a sensor, it should be a total bromine sensor.
References
1 Goosan, M. F. A. & Shayra, W. H. Water management, purification, and conservation in arid climates. Volume 2: Water purification. (Univ. of Sultan Qaboos Univ.(OM), 1999).
2 Wiley, White’s Handbook of Chlorination and Alternative Disinfectants, 5th Edition. (page 874, pages 122-129).
3 Wieser, M. E. et al. Atomic weights of the elements 2011 (IUPAC Technical Report). Pure Appl. Chem. 85, 1047-1078 (2013).
Measuring free chlorine and chlorine dioxide independently of each other is quite a challenge, given their chemical similarities. Many sensors struggle to differentiate between the two measurands, but did you know that…
…many chlorine probes suffer from interference in the presence of chlorine dioxide?
…DPD1 will read both chlorine and chlorine dioxide?
…you can have accurate chlorine dioxide control in water where chlorine is present?
Free chlorine and chlorine dioxide are both oxidants used for disinfection in water. Each act differently as a disinfectant but are measured in almost the same way; with an electrochemical sensor or with an online DPD sensor. It is sometimes beneficial to have both disinfectants in the water at the same time, particularly when chlorine dioxide is being added to mains water.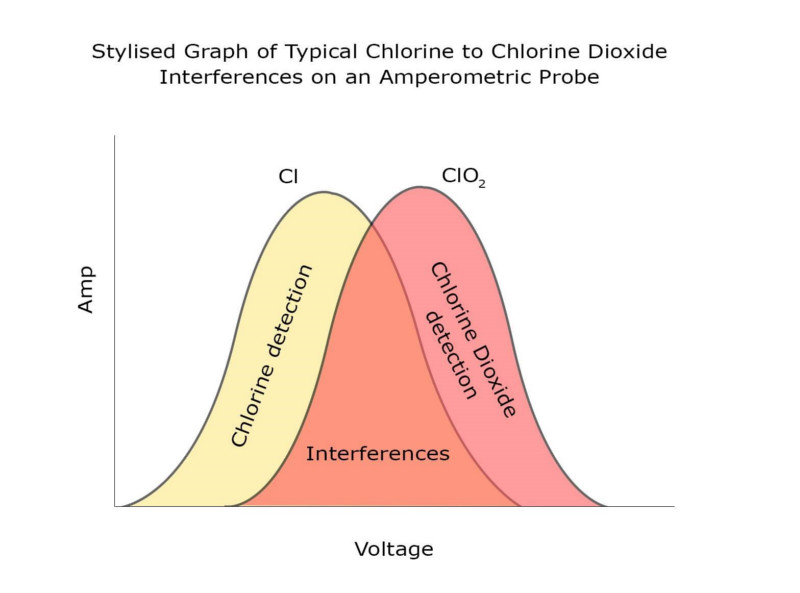 In most non-membraned (and some membraned) amperometric sensors, oxidants are detected by a current produced at the working electrode, at a particular voltage. The same technology can effectively be ‘tuned’ to different oxidisers by varying the voltage. Lots of oxidants are measurable over a range of voltages, and sometimes those response curves overlap.
In most non-membraned (and some membraned) amperometric sensors, oxidants are detected by a current produced at the working electrode, at a particular voltage. The same technology can effectively be ‘tuned’ to different oxidisers by varying the voltage. Lots of oxidants are measurable over a range of voltages, and sometimes those response curves overlap.
The graph shows that at almost any voltage where you can measure free chlorine, the chlorine dioxide curve overlaps with the chlorine one. This means it can be very difficult to find a probe that measures free chlorine, but doesn’t measure chlorine dioxide. Although these response curves can shift depending on probe design, electrode material and other factors, it is very difficult to engineer a response curve that gives a good signal for free chlorine but not for chlorine dioxide. The DioSense membraned chlorine dioxide sensor from Pi is not susceptible to interference by free chlorine. This means that the DioSense sensor can be used in conjunction with Pi’s HaloSense free chlorine sensor, to measure chlorine dioxide and free chlorine independently of each other in the same application, on Pi’s CRONOS® or CRIUS® analyser. The analyser takes the signal from the free chlorine probe, which does have a known interference from chlorine dioxide (1ppm of chlorine dioxide will show up as 0.75ppm of chlorine). The normalised signal from the chlorine dioxide sensor can then be removed.
The DioSense membraned chlorine dioxide sensor from Pi is not susceptible to interference by free chlorine. This means that the DioSense sensor can be used in conjunction with Pi’s HaloSense free chlorine sensor, to measure chlorine dioxide and free chlorine independently of each other in the same application, on Pi’s CRONOS® or CRIUS® analyser. The analyser takes the signal from the free chlorine probe, which does have a known interference from chlorine dioxide (1ppm of chlorine dioxide will show up as 0.75ppm of chlorine). The normalised signal from the chlorine dioxide sensor can then be removed.
If you’ve ever had an application that uses Reverse Osmosis (RO), such as in a renal ward, you probably understand the damaging effect that chlorine can have on RO membranes, but did you know that…
…Pi has a security system that can alert you to any free chlorine present and can actually prevent the contaminated water from reaching the membranes at all?
The most common source of water for corporate buildings, hospitals and industrial processes, is standard mains water. This water typically contains chlorine, added by municipal water companies, for its disinfection properties. For any system that requires RO membranes, if the water source is mains water, it is common to have carbon filters positioned before the membranes. The carbon filters are designed to remove any chemicals in the water that might be damaging to the RO membranes, including chlorine. Our HaloSense Zero chlorine controller measures the water coming out of the carbon filters before it reaches the RO membranes. Any free chlorine breakthrough from the carbon filters is detected by the system, which will set off an alarm and can also be configured to automatically shut off valves to prevent the contaminated water from reaching the RO membranes.
Our HaloSense Zero chlorine controller measures the water coming out of the carbon filters before it reaches the RO membranes. Any free chlorine breakthrough from the carbon filters is detected by the system, which will set off an alarm and can also be configured to automatically shut off valves to prevent the contaminated water from reaching the RO membranes.
The HaloSense Zero effectively acts as a security measure to ensure that if there is a breakthrough of chlorine, the system can be shut down to prevent damage to the RO membranes. This can help to extend the lifetime of the expensive RO membranes and save the customer money.
The removal of chlorine is even more important in hospital systems where the water is supplied to renal wards. In this case the HaloSense Zero chlorine analyser not only protects the RO membranes, but it can also help to save lives. If any chlorine was to break through the carbon filters and make it past the RO membranes as well, it could endanger the lives of renal patients in the hospital. The HaloSense Zero chlorine system is a fantastic safety feature, providing an extra line of defence against the potential hazard. The HaloSense Zero consists of a sensor designed to measure the absence of chlorine connected to a CRONOS® or CRIUS® controller. The sensor can detect even very low levels of free chlorine, while the CRONOS® or CRIUS® controller acts as the brains of the system, interpreting data from the sensor. In the event that any free chlorine is detected, the analyser will send an alarm signal and can automatically shut off valves to prevent chlorine from reaching the RO membranes.
The HaloSense Zero consists of a sensor designed to measure the absence of chlorine connected to a CRONOS® or CRIUS® controller. The sensor can detect even very low levels of free chlorine, while the CRONOS® or CRIUS® controller acts as the brains of the system, interpreting data from the sensor. In the event that any free chlorine is detected, the analyser will send an alarm signal and can automatically shut off valves to prevent chlorine from reaching the RO membranes.
So why aren’t you using this system already? Are there any catches?
The only catch is that the HaloSense Zero can’t measure combined chlorines in the water. Typical mains water in most countries contains a mixture of free chlorine and combined chlorine which is what the HaloSense Zero is designed to detect and help protect the RO membranes against.
The ingenious HaloSense Zero chlorine analyser will even periodically check the responsiveness of the sensor automatically, to ensure that the sensor is still reacting correctly when free chlorine is present. It does this by switching between the post-carbon filter water and chlorinated mains water using a 3-way solenoid valve controlled by a programmed timer.
If you are using a carbon filter before your RO membranes to remove the chlorine, probably the only reason you aren’t using this system already is simply that you haven’t heard of it before. The Pi HaloSense Zero chlorine system is just one of Pi’s innovative solutions to water treatment problems.
Many different sites ranging across the whole water industry have a daily struggle to keep instrumentation functioning correctly due to fouling. However, did you know that…
…self cleaning and self flushing systems are now available from Process Instruments for most types of sensors?
…these fouling removal systems can extend the life of sensors and drastically reduce maintenance regimes?
…Pi’s self cleaning/flushing systems are affordable, simple and trouble free by design?
Sensor Fouling
 Whatever the process being monitored is, there is often something in the sample water capable of fouling a sensor, and therefore causing erroneous results. The obvious solution to this problem is to clean the sensor, but how regular should inspection and cleaning programs be for each piece of instrumentation? Too regular and the inspection and cleaning regime is time consuming and unnecessarily costly. Not often enough and the instrumentation will give false results and probably fail prematurely.
Whatever the process being monitored is, there is often something in the sample water capable of fouling a sensor, and therefore causing erroneous results. The obvious solution to this problem is to clean the sensor, but how regular should inspection and cleaning programs be for each piece of instrumentation? Too regular and the inspection and cleaning regime is time consuming and unnecessarily costly. Not often enough and the instrumentation will give false results and probably fail prematurely.
What is the solution?
Process Instruments’ AutoClean and AutoFlush Systems
Simple, reliable and easy to maintain, Process Instruments’ AutoClean/AutoFlush systems are an alternative to mechanical cleaning mechanisms which can clog and break. By regularly spraying the sensor/probe with clean water or air, the sensor remains clean and free from fouling for extended periods of time. The sensor cleaning cycle is activated by Pi’s controller for a user selectable length of time and frequency so that no matter how dirty the application, the probe remains clean. With no moving parts in the sensor body or in the cleaning attachment there is nothing to replace or check other than a simple valve positioned in an easy to reach location. Pi’s AutoClean and AutoFlush systems can give trouble free and fouling free functioning of sensors for weeks, if not months, at a time.
Pi’s AutoClean and AutoFlush systems can give trouble free and fouling free functioning of sensors for weeks, if not months, at a time.
A solution for each application
AutoClean
This option can be added to our pH, ORP, Turbidity, Suspended Solids and Dissolved Oxygen (DO) sensors. Consisting of an end cap to direct the flow of clean water (or air for a DO sensor) across the face of the sensor blasting any dirt away. The cleaning is controlled by a single valve positioned in an easily accessible location.
AutoVerify
 If using air to clean a DO sensor the system can also automatically verify that the sensor is still responding correctly, removing any need to remove the sensor from the sample for months at a time.
If using air to clean a DO sensor the system can also automatically verify that the sensor is still responding correctly, removing any need to remove the sensor from the sample for months at a time.
AutoFlush
For sensors that require flow cell mounting like Chlorine, Ozone and Chlorine Dioxide, an Autoflush system has inbuilt valves which automatically start/stop the sample flow and control the flow of clean water past the probe. The user can set the flushing interval and duration to keep the flow cell and sensor clear from fouling. For particularly dirty or stubborn contaminants, warm water can be used as the flush water to aid cleaning.
With the above options, whatever the application or parameter being measured, Process Instruments will be able to provide a monitoring system that will not only be accurate, precise and long lasting but that will also remain free from fouling and save the operator both time and money.
You probably know that most chlorine, ozone and chlorine dioxide analysers are calibrated using hand held DPD kits but did you know that…
…DPD can’t tell you when you have no residual?
…errors on DPD performance can be up to ± 100%?
…a significant number of service calls received by Pi relate to poor calibration?
What is DPD?
DPD (N.N-diethyl-p-phenylenediamine) is a chemical that when mixed with water containing an oxidant, changes colour depending on the concentration of the oxidant present. A handheld colorimeter measures light passing through the coloured solution. The absorption of that light by the liquid gives a concentration value. It is usually used to check concentration of, for example, free chlorine, total chlorine, ozone and chlorine dioxide etc. in water. When the DPD kit gives a value, it is often used to calibrate online instruments… and that is where Pi comes in!
When the DPD kit gives a value, it is often used to calibrate online instruments… and that is where Pi comes in!
As a manufacturer of online instruments we have to understand DPD in order to help our customers when they have problems calibrating their online monitors.
This Focus On will look at:
- The limitations of DPD (turbidity, zero oxidant, bleaching, pH and interferents).
- Minimising DPD measurement error (sampling, alignment and cleaning).
- Things to look out for (low concentrations, pink colour, stained glass).
- Little known chemistry (measuring bromine, chlorite versus chlorine dioxide).
- Rinse and repeat: is it really worth repeating my measurement?
What are the limitations of DPD?
DPD cannot measure below approximately 0.05 ppm.
 If you suspect there is zero oxidant in your sample, hold the vial up to a white surface. If you cannot see any trace of pink colour, it is likely any reading you are getting is from the unreacted DPD tablet.
If you suspect there is zero oxidant in your sample, hold the vial up to a white surface. If you cannot see any trace of pink colour, it is likely any reading you are getting is from the unreacted DPD tablet.
DPD cannot measure free chlorine above 6ppm
(and won’t always give a ‘high concentration’ reading error).
Many people are unaware that past a certain level of oxidant, DPD will not form its characteristic pink colour, and instead will ‘bleach’ to form a clear solution. This can lead people to think there is little or no oxidant in their water, when in fact there is so much that it is bleaching their DPD. Be on the lookout for a flash of pink when the tablet or powder is added if you suspect your sample is being bleached. NB. special kits and reagents are available for measuring oxidant above 6 ppm.
DPD cannot distinguish between oxidants such as:
chlorine, chlorine dioxide, chlorite, ozone, organochlorides, bromine and more, meaning interferents are a big problem.
DPD is a fantastic chemical, in that it is very versatile as a colouring agent, which is how it gives the oxidant the colour that we measure. This versatility does come at a price, DPD is not very specific as an analysis tool, and so if other chemicals are present in the sample, they can interfere with the reading, giving an inaccurate result. Common interferents include chlorine dioxide (for chlorine measurement, and vice versa), sodium chlorite, ozone, organochloramines, peroxides, and many more.
Minimising DPD measurement error
Here is an easy to read, printable checklist to ensure accurate DPD readings every time.
What To Watch Out for When Using DPD
Stained Glass
 The pink solution formed after DPD tests can leave a residue behind on the glass, which will affect the DPD reading. This residue can be easily cleaned off using what is in your DPD kit.
The pink solution formed after DPD tests can leave a residue behind on the glass, which will affect the DPD reading. This residue can be easily cleaned off using what is in your DPD kit.
Tap water
If you use normal tap water to wash out vials, droplets left behind can affect your reading due to the residual chlorine in drinking water. It is best (but not always practical) to use deionised water to wash out your vials, but if this isn’t available (deionised water can be purchased as car battery top up water from any car parts supplier) then you can use cooled boiled tap water, as boiling gets rid of any chlorine. If not then simply make sure the vials are perfectly dry before use.
Little Known Chemistry
DPD has a wide range of interferents. This means recurrent problems can sometimes be caused by the chemical makeup of the sample. For example, chlorite (ClO2–) and chlorine dioxide both affect DPD, but only chlorine dioxide is measured by most chlorine dioxide amperometric sensors.
DPD can be used to track bromine, but DPD No.1 tablets measure FREE chlorine or TOTAL bromine. As combined bromine is just as effective a disinfectant as free bromine, this generally doesn’t pose too much of a problem, however some amperometric sensors measure free bromine, and cannot be calibrated using DPD No.1 tablets. For more information on measuring bromine, or chlorine in seawater, please see Pi’s technical note on Seawater Chlorination.
You probably know that some instruments use ORP to control chlorine dosing and others use ppm chlorine sensors but did you know that…
…ORP over about 3ppm won’t work?
…swimming pools in the USA use ORP and in Europe use ppm chlorine sensors?
…the ORP of towns water can vary a great deal? In the USA nearly all pools and spas use ORP sensors to control their chlorine dose, yet conversely in the UK and Western Europe most ORP systems have been replaced with systems that measure the concentration of free chlorine in water. Pi provides systems that utilise either or both technologies.
In the USA nearly all pools and spas use ORP sensors to control their chlorine dose, yet conversely in the UK and Western Europe most ORP systems have been replaced with systems that measure the concentration of free chlorine in water. Pi provides systems that utilise either or both technologies.
ORP
Oxidation reduction potential (ORP or REDOX) sensors, measure the tendency of water to gain or lose electrons from anything in the water. The more positive a reading from an ORP the greater the tendency the water has to oxidise (gain electrons from) organisms or other material in the water, thereby killing or destroying them.
Why do so many pools in the USA use ORP?
When chlorine is dosed into a pool it form OCl– and HOCl. Disinfection is largely done by the HOCl and ORP responds to the concentration of HOCl in the water, which makes it a good measure of the tendency of the chlorine in the water to kill bugs. Despite this, ORP is a secondary measure of HOCl and is affected by a multitude of other factors, some of which will be touched on below. The main attractions of ORP are; low purchase cost, no calibration and little or no maintenance.
What are the problems with ORP sensors?
 Unfortunately, what ORP sensors measure is tendency and not capacity, i.e. ORP measures the likelihood or the ability of the water to kill bugs, but not how many bugs that water can kill, a subtle but very important difference. A sample with high ORP may be able to kill a small number of bugs very quickly but then not be able to kill future pollution. What’s more, although chlorine affects ORP very strongly it is not the only variable involved. The pH of water affects ORP directly and also affects the concentration ratio of OCl–/HOCl, the two main disinfectant components. A lower pH (higher acidity) will cause an increase in the relative concentrations of HOCl causing an increase in ORP.
Unfortunately, what ORP sensors measure is tendency and not capacity, i.e. ORP measures the likelihood or the ability of the water to kill bugs, but not how many bugs that water can kill, a subtle but very important difference. A sample with high ORP may be able to kill a small number of bugs very quickly but then not be able to kill future pollution. What’s more, although chlorine affects ORP very strongly it is not the only variable involved. The pH of water affects ORP directly and also affects the concentration ratio of OCl–/HOCl, the two main disinfectant components. A lower pH (higher acidity) will cause an increase in the relative concentrations of HOCl causing an increase in ORP.
Perhaps the biggest issue with ORP is that the ORP readings on water with no chlorine in it will be different depending on the source of that water. This means that an ORP of 750mV in one part of the country is not the same chlorine concentration as 750mV in another part of the country. Also the ORP response to HOCl is not linear and increasing residual chlorine above 3 ppm has little effect on ORP readings making control above 3 ppm extremely difficult. These issues typically lead to overdosing the water with chlorine, in order to compensate for these effects. This can be seen very clearly in US pools which often have more than 2 ppm of chlorine compared to European pools which typically operate around 0.8-1.5 ppm (The World Health Organisation recommends 1 ppm residual).
ppm Chlorine
 These sensors use electrochemistry to measure the free chlorine concentration directly. They tend to be slightly more expensive than an ORP sensor, but are more reproducible and precise, and therefore tend to give better control (and therefore reduced chemical cost). They are specific to free chlorine (the disinfectant) and can be easily calibrated using a DPD test for free chlorine. Whilst the capital cost for a ppm chlorine sensor is higher, total cost of ownership tends to be lower as ORP sensors are typically replaced every year and ppm sensors last for ten years or more.
These sensors use electrochemistry to measure the free chlorine concentration directly. They tend to be slightly more expensive than an ORP sensor, but are more reproducible and precise, and therefore tend to give better control (and therefore reduced chemical cost). They are specific to free chlorine (the disinfectant) and can be easily calibrated using a DPD test for free chlorine. Whilst the capital cost for a ppm chlorine sensor is higher, total cost of ownership tends to be lower as ORP sensors are typically replaced every year and ppm sensors last for ten years or more.
Problems with ppm Chlorine sensors
A ppm sensor measures the capacity of water to kill organisms, the only problem is that it doesn’t measure how fast the bugs are killed, a variable largely down to pH. There are two different types of ppm sensors. The first measure only HOCl, and have very similar problems to ORP sensors. The other type of sensor, in pHs below 8.0, measure both HOCl and OCl–. Pi only recommends the use of sensors that (for use in pools) are independent of pH, and the use of pH control that is independent of chlorine dosage. This leads to tighter control of both pH and free chlorine meaning chlorine residuals can be more tightly controlled and reduced, which in turn leads to lower costs and a more pleasant bathing experience.
Conclusion
Advantages | Disadvantages |
|---|---|
ORP SensorsSimple (no calibration)Inexpensive | ORP SensorsDoesn’t measure disinfection capacityAffected more by pH than by free chlorine Non-Linear Not reproducible (not the same from site to site) Affected by changing water chemistry Affected by all oxidants Using ORP control normally leads to higher residuals and less stable control |
ppm SensorsMeasure free chlorine directlyResults comparable across different sites Linear response Only affected by free chlorine Using a ppm sensors leads to lower residuals, more stable control and better swimmer experiences | ppm SensorsRequires calibrationMore expensive – but not much More maintenance – but not much |
| Document | Type | Size |
|---|---|---|
| HaloSense | Brochure | 724kB |
| HaloSense Zero | Technical Note | 606kB |
| HaloSense Hints and Tips | Technical Note | 606kB |
| Potential Savings when Choosing Amperometric Chlorine Measurements over online DPD | Article | 525kB |
| Seawater Chlorination | Technical Note | 710kB |
| ORP vs. ppm | Technical Note | 534kB |
| pH Effects on Pi’s Free Chlorine Sensor | Technical Note | 702kB |
| pH Compensation of a Residual Chlorine Measurement | Technical Note | 540kB |
| Free Chlorine Probe Maintenance | Technical Note | 658kB |
| Total Chlorine Probe Maintenance | Technical Note | 689kB |
| Using Open Flow Cells with Membraned Sensors | Technical Note | 762kB |
| DPD Checklist | Technical Note | 487kB |
| Probe Fouling | Technical Note | 459kB |
Click on any of the buttons below to see of product details
"The support from Pi and its partners is superb. They go above and beyond to ensure that, not only is their equipment perfect but that the process is working great too. Five Stars!"
Anthony Glitto
Equip Solutions - Illinois, USA
Everyone at Pi goes out of their way to be supportive and timely. Their expertise is unchallenged and I am very happy to endorse them as a quality supplier.
John Clark
Chemtrac - Georgia, USA
Servicing customers is much more than just solving problems or addressing complaints and Pi does that very competently with technical and quick efforts providing a good experience.
Clovis Tuchapski
Buckman - Latin America









